1pure subtance xis solid at room temerature. if the subtance is heated to230 c is melted gradually. if then cooled to room temperatur, the liquid cat not be frozen
a is it possible xmof an element or acmpound explain it
answer a element because liquid subtance is heate to temparatur combination of elemnt chemical the properties of apure subtance make it possible to identify that subtance concusively
b yes because chang chemical undeago an endotrem
c cen it be said that the liqid is an element because pure subtane x is solid at rooom temperature and 230 c is heated melted gradually chemical undergo an endotem
2when a candlle that weighs 10 g is burned in oxygen carbo dioxide and water vapor formed by combinatian the weigh more that 10 g .was this case mtch with the law of concerfation of mass examlain it
answer: that the compound containing atoms of different elemets era joined with a rarion of intgres not destroyed chemical reaction
3when carbon burns in oxygen under limited number , it will form two gaseous compouns .suggestbthe way to differentiate the two compouns with one anorher answer: to differentiate the two compounds carbon burns in oxygen undr limited number the same proporitions and other pure subtance
4 answer because the anderstanding of peridic regularity in physical and chemical behavior and the need for mangorganir all bout the structure and properties of elemnt has led to the blossoms periodic fable
5 answer aqueos solution of merrcury cloride of siler nitrat has the ability to conduct electricity when the solute is an electrolyte reaction
Senin, 28 November 2011
Jumat, 11 November 2011
Stoichiometry ( /ˌstɔɪkiˈɒmɨtri/) is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers. For example, in a reaction that forms ammonia (NH3), exactly one molecule of nitrogen (N2) reacts with three molecules of hydrogen (H2) to produce two molecules of NH3:
/ˌstɔɪkiˈɒmɨtri/) is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers. For example, in a reaction that forms ammonia (NH3), exactly one molecule of nitrogen (N2) reacts with three molecules of hydrogen (H2) to produce two molecules of NH3:
- N2 + 3H2 → 2NH3
Stoichiometry can be used to calculate quantities such as the amount of products (in mass, moles, volume, etc.) that can be produced with given reactants and percent yield (the percentage of the given reactant that is made into the product). Stoichiometry calculations can predict how elements and components diluted in a standard solution react in experimental conditions. Stoichiometry is founded on the law of conservation of mass: the mass of the reactants equals the mass of the products.
Reaction stoichiometry describes the quantitative relationships among substances as they participate in chemical reactions. In the example above, reaction stoichiometry describes the 1:3:2 ratio of molecules of nitrogen, hydrogen, and ammonia.
Composition stoichiometry describes the quantitative (mass) relationships among elements in compounds. For example, composition stoichiometry describes the nitrogen to hydrogen (mass) relationship in the compound ammonia: i.e., one mole of nitrogren and three moles of hydrogen are in every mole of ammonia.
A stoichiometric amount or stoichiometric ratio of a reagent is the optimum amount or ratio where, assuming that the reaction proceeds to completion:
- all reagent is consumed,
- there is no shortfall of reagent, and
- no residues remain.
A non-stoichiometric mixture, where reactions have gone to completion, will have only the limiting reagent consumed completely.
While almost all reactions have integer-ratio stoichiometry in amount of matter units (moles, number of particles), some nonstoichiometric compounds are known that cannot be represented by a ratio of well-defined natural numbers. These materials therefore violate the law of definite proportions that forms the basis of stoichiometry along with the law of multiple proportions.
Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume, and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.
The elementary reaction is the smallest division into which a chemical reaction can be decomposed to, it has no intermediate products.[9] Most experimentally observed reactions are built up from many elementary reactions that occur in parallel or sequentially. The actual sequence of the individual elementary reactions is known as reaction mechanism. An elementary reaction involves a few molecules, usually one or two, because of the low probability for several molecules to meet at a certain time.[10]
The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by an isomerization or a dissociation in one or more other molecules. Such reaction requires addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–transisomerization, in which the cis-form of a compound converts to the trans-form or vice versa.[11]
In a typical dissociation reaction, a bond in a molecule splits resulting in two molecular fragments. The splitting can be homolyticor heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ions. Dissociation plays an important role in triggering chain reactions, such as hydrogen–oxygen or polymerization reactions.
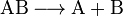
- Dissociation of a molecule AB into fragments A and B
For bimolecular reactions, two molecules collide and react with each other. Their merger is called chemical synthesis or an addition reaction.
Another possibility is that only a portion of one molecule is transferred to the other molecule. This type of reaction occurs, for example, in redox and acid-base reactions. In redox reactions, the transferred particle is an electron, whereas in acid-base reactions it is a proton. This type of reaction is also called metathesis.
for example
[edit]Chemical equilibrium
Main article: Chemical equilibrium
Most chemical reactions are reversible, that is they can and do run in both directions. The forward and reverse reactions are competing with each other and differ in reaction rates. These rates depend on the concentration and therefore change with time of the reaction: the reverse rate gradually increases and becomes equal to the rate of the forward reaction, establishing the so-called chemical equilibrium. The time to reach equilibrium depends on such parameters as temperature, pressure and the materials involved, and is determined by the minimum free energy. In equilibrium, the Gibbs free energy must be zero. The pressure dependence can be explained with the Le Chatelier's principle. For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with the fewer moles of gas.[12]
The reaction yield stabilized at equilibrium, but can be increased by removing the product from the reaction mixture or increasing temperature or pressure. Change in the initial concentrations of the substances does not affect the equilibrium.
[edit]Thermodynamics
Chemical reactions are determined by the laws of thermodynamics. Reactions can proceed by themselves if they are exergonic, that is if they release energy. The associated free energy of the reaction is composed of two different thermodynamic quantities, enthalpy and entropy:[13]

- G: free energy, H: enthalpy, T: temperature, S: entropy, Δ: difference
Reactions can be exothermic, where ΔH is negative and energy is released. Typical examples of exothermic reactions are precipitation and crystallization, in which ordered solids are formed from disordered gaseous or liquid phases. In contrast, in endothermic reactions, heat is consumed from the environment. This can occur by increasing the entropy of the system, often through the formation of gaseous reaction products, which have high entropy. Since the entropy increases with temperature, many endothermic reactions preferably take place at high temperatures. On the contrary, many exothermic reactions such as crystallization occur at low temperatures. Changes in temperature can sometimes reverse the direction of a reaction, as in the Boudouard reaction:
This reaction between carbon dioxide and carbon to form carbon monoxide is endothermic at temperatures above approximately 800 °C and is exothermic below this temperature.[14]
Reactions can also be characterized with the internal energy which takes into account changes in the entropy, volume and chemical potential. The latter depends, among other things, on the activities of the involved substances.[15]
Langganan:
Postingan (Atom)




