The elementary reaction is the smallest division into which a chemical reaction can be decomposed to, it has no intermediate products.[9] Most experimentally observed reactions are built up from many elementary reactions that occur in parallel or sequentially. The actual sequence of the individual elementary reactions is known as reaction mechanism. An elementary reaction involves a few molecules, usually one or two, because of the low probability for several molecules to meet at a certain time.[10]
The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by an isomerization or a dissociation in one or more other molecules. Such reaction requires addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–transisomerization, in which the cis-form of a compound converts to the trans-form or vice versa.[11]
In a typical dissociation reaction, a bond in a molecule splits resulting in two molecular fragments. The splitting can be homolyticor heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ions. Dissociation plays an important role in triggering chain reactions, such as hydrogen–oxygen or polymerization reactions.
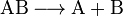
- Dissociation of a molecule AB into fragments A and B
For bimolecular reactions, two molecules collide and react with each other. Their merger is called chemical synthesis or an addition reaction.
Another possibility is that only a portion of one molecule is transferred to the other molecule. This type of reaction occurs, for example, in redox and acid-base reactions. In redox reactions, the transferred particle is an electron, whereas in acid-base reactions it is a proton. This type of reaction is also called metathesis.
for example
[edit]Chemical equilibrium
Main article: Chemical equilibrium
Most chemical reactions are reversible, that is they can and do run in both directions. The forward and reverse reactions are competing with each other and differ in reaction rates. These rates depend on the concentration and therefore change with time of the reaction: the reverse rate gradually increases and becomes equal to the rate of the forward reaction, establishing the so-called chemical equilibrium. The time to reach equilibrium depends on such parameters as temperature, pressure and the materials involved, and is determined by the minimum free energy. In equilibrium, the Gibbs free energy must be zero. The pressure dependence can be explained with the Le Chatelier's principle. For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with the fewer moles of gas.[12]
The reaction yield stabilized at equilibrium, but can be increased by removing the product from the reaction mixture or increasing temperature or pressure. Change in the initial concentrations of the substances does not affect the equilibrium.
[edit]Thermodynamics
Chemical reactions are determined by the laws of thermodynamics. Reactions can proceed by themselves if they are exergonic, that is if they release energy. The associated free energy of the reaction is composed of two different thermodynamic quantities, enthalpy and entropy:[13]

- G: free energy, H: enthalpy, T: temperature, S: entropy, Δ: difference
Reactions can be exothermic, where ΔH is negative and energy is released. Typical examples of exothermic reactions are precipitation and crystallization, in which ordered solids are formed from disordered gaseous or liquid phases. In contrast, in endothermic reactions, heat is consumed from the environment. This can occur by increasing the entropy of the system, often through the formation of gaseous reaction products, which have high entropy. Since the entropy increases with temperature, many endothermic reactions preferably take place at high temperatures. On the contrary, many exothermic reactions such as crystallization occur at low temperatures. Changes in temperature can sometimes reverse the direction of a reaction, as in the Boudouard reaction:
This reaction between carbon dioxide and carbon to form carbon monoxide is endothermic at temperatures above approximately 800 °C and is exothermic below this temperature.[14]
Reactions can also be characterized with the internal energy which takes into account changes in the entropy, volume and chemical potential. The latter depends, among other things, on the activities of the involved substances.[15]





Tidak ada komentar:
Posting Komentar